Last week you measured enzyme kinetics parameters Vmax, Km, and Vmax/Km. The reason for measuring such parameters is usually to see how they change with respect to some variable, such as pH, different substrates, and different inhibitor concentrations. Such studies are how biochemists discover the way that enzymes and inhibitors work.
Inhibitors are chemicals that decrease enzyme activity. The study of enzyme inhibitors can yield valuable information concerning the mechanism of enzyme action and structure of the active site. Knowledge of inhibition mechanism is also useful for the design of affinity chromatography materials for selective protein purification and for the design of new types of drugs (many drugs are competitive inhibitors of enzymes). The following discussion will highlight the many different ways that inhibitors can function.
Lecture Video
Irreversible Inhibitors
Irreversible inhibitors (Hg, Pb, Cd, nerve gases, etc.) usually bind to a specific amino acid residue in the enzyme and block catalysis either by modifying the active site or disturbing the conformation of the protein. These inhibitors are commonly called poisons. Irreversible inhibitors function in a time-dependent manner such that the rate of enzyme reaction decays exponentially with time.
Reversible Inhibitors
In contrast, reversible inhibitors function in a concentration-dependent manner. Their mechanisms of action are elucidated by observing their effects on Vmax/Km (kcapture), Vmax (krelease).
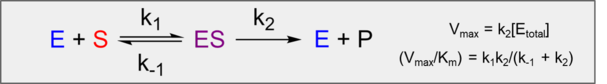

Identification and classification of reversible inhibitors into various categories is classically accomplished using enzyme kinetics data and graphical methods of analysis. The manner and degree to which these parameters are affected is apparent from changes in slope and/or intercept in various linear transforms: Lineweaver-Burk, Eadie-Hofstee, Direct Linear, or Hanes-Woolf plots. Although, any graphical analysis method may be used, most authors and textbooks still present Lineweaver-Burk plots. However, since the Lineweaver-Burk plot yields poor estimates of kinetic constants, we will use the Eadie-Hofstee transform to determine our apparent kinetics constants in the presence of inhibitor. Once the kinetic mechanism is classified, the inhibition data can be used to calculate the apparent inhibitor dissociation constant, Ki. A discussion of the major categories of reversible inhibitors follows.
Competitive Inhibitors
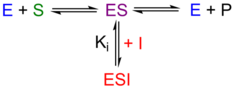
Competitive inhibitors bind to the free enzyme at the active site. They have a structure similar enough to the substrate to become a substrate "look-alike." Because of their ability to fit into the active site, catalysis is blocked.

The equilibrium constant for inhibitor binding, defined as Ki = [E][I]/[EI], modifies the apparent Vmax/Km (also known as kcapture, the rate of substrate capture into the ES complex) but not the Vmax (also known as krelease, the rate of release from the ES complex). Increasing the substrate concentration can overcome inhibition caused by a competitive inhibitor. Measurement of vo at various concentrations of [S] in the presence of a constant amount of competitive inhibitor yields an Eadie-Hofstee plot similar to that in Figure 1.
Once the mechanism is confirmed to be competitive inhibition, the equilibrium constant Ki may be calculated from the smaller apparent Vmax/Km in the presence of inhibitor using the relationship in Equation 1,
$$ \left( \frac{V_{\text{max}}}{K_m} \right)_{\text{app}} = \left( \frac{V_{\text{max}}}{K_m} \right) \bigg/ \left( 1 + \frac{[\text{I}]}{K_i} \right) \tag{1} $$where (Vmax/Km) is that kinetic parameter measured without inhibitor, (Vmax/Km)app is the apparent (Vmax/Km) in the presence of inhibitor at concentration [I], and Ki is the dissociation constant for the inhibitor binding to the free enzyme. When interpreting the Ki for competitive inhibition, it is worth noting that this parameter should match a published literature value for the same inhibitor, enzyme, and conditions regardless of the enzyme substrate used. Competitive inhibitors bind the free enzyme, therefore the choice of substrate should have no impact.
Uncompetitve Inhibitors
Pure uncompetitive inhibitors are relatively rare. This type of inhibitor binds only to the ES complex. Since the rate of capture into the ES complex is not affected the presence of inhibitor does not change Vmax/Km, but it does lower the apparent Vmax, the rate of release from the ES complex.
Measurement of vo at various concentrations of [S] in the presence of an uncompetitive inhibitor yields a Eadie-Hofstee plot similar to that of Figure 3.
The equilibrium constant for inhibitor binding is defined as Ki = [ES][I]/[ESI]. The equilibrium constant may be calculated from the apparent Vmax in the presence of inhibitor from Equation 2,
$$ (V_{\text{max}})_{\text{app}} = V_{\text{max}} \bigg/ \left( 1 + \frac{[\text{I}]}{K_i} \right) \tag{2} $$where Vmax is that kinetic parameter measured without inhibitor, (Vmax)app is the apparent Vmax in the presence of inhibitor at concentration [I], and Ki is the dissociation constant for the inhibitor binding to the enzyme-substrate complex. When interpreting the Ki for uncompetitive inhibition, it is worth noting that this parameter should may be dependent on the substrate used and a reference value from the literature acquired with a different enzyme substrate may not necessarily have the same inhibitor dissociation constant.
Mixed Inhibitors
Mixed inhibitors can bind to the free enzyme or the ES complex. Usually this binding takes place at some site other than the active site, but binding at this site does affect binding at the catalytic site. In contrast to competitive inhibition, increasing the substrate concentration cannot overcome this type of inhibition. Mixed inhibitors usually exert a greater effect on Vmax, but affect Vmax/Km as well.
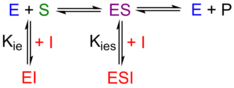
In mixed inhibition, the equilibrium constant for inhibitor binding, Ki, is composed contributions from (competitive) inhibition of substrate capture, Kie = [E][I]/[EI], and (uncompetitive) inhibition of catalysis and/or product release from the ES complex, Kies = [ES] [I] / [ESI]. Inhibition constant Kie, may be calculated from the apparent Vmax/Km in the presence of inhibitor from the Equation 3.
$$ \left(\frac{V_{max}}{K_m}\right)_{app} = \left(\frac{V_{max}}{K_m}\right) \Bigg/ \left(1 + \frac{[I]}{K_{ie}}\right) \tag{3} $$The constant Kies may be calculated from the apparent Vmax using Equation 4.
$$ (V_{max})_{app} = V_{max} \Bigg/ \left(1 + \frac{[I]}{K_{ies}}\right) \tag{4} $$Measurement of vo at various concentrations of [S] in the presence of mixed inhibitors yields an Eadie-Hofstee plot similar to that of Figure 2. On rare occasions, a special case of mixed inhibition, called "noncompetitive inhibition" occurs when the inhibitor has equal affinity for E and ES (the dissociation equilibrium constants are equal). For this special case, Ki = Kie = Kies. Noncompetitive inhibitors typically bind to a site that prevents the enzyme from catalyzing its reaction without actually interfering with the substrate binding site.
Based upon the line patterns in the Eadie-Hofstee plots, a guess can be made as to what type of inhibitor is affecting an enzyme. Keep in mind that the plots above are "ideal," and the type of inhibition is usually not so obvious for real experimental data. Also, you may have noticed that textbooks typically discuss these trends in terms of the effects of inhibitor on Km. However, it is much simpler to understand these constants in terms of the effects of their effects on capture into and release from the ES complex, represented by the rate constants (Vmax/Km) and Vmax respectively, which are in turn expressed by the x-intercept and y-intercepts of the Eadie-Hofstee plot.
Tyrosinase Inhibitors
Tyrosinase is a copper containing oxido-reductase. Over the years, many compounds have been found that inhibit the enzyme in some manner. Some of the inhibitors are enzymatic poisons while others act to reduce product quinones back to phenolic substrates. Others can act as copper chelators, removing the essential active-site copper ions. Some of the inhibitors act in a competitive manner, some in a noncompetitive manner, while others display mixed inhibition. In general, tyrosinase inhibitors fall into two categories: those that affect the active-site copper ions, and those that affect the phenolic/substrate binding site. Since there is an oxygen binding site, molecules such as CO and CO2 also inhibit tyrosinase much like they do in cellular respiration. The table below gives a partial list of tyrosinase inhibitors. Many of these inhibitors act in multiple ways to inhibit the enzyme, so they appear in more than one category below. Literally, hundreds of tyrosinase inhibitors have been reported in the literature.
|
Enzyme binding |
Cu chelators |
Reducing agents |
Quinone conjugators |
Miscellaneous |
|
hydroxamic acid |
EDTA |
ascorbate |
methimazole |
polyvinylpyrrolidone |
|
cinnamic acid |
diethyldithiocarbamate |
mercaptoethanol |
kojic acid |
H2O2 |
|
p-coumaric acid |
phenylthiourea |
bisulfite |
cysteine |
honey |
|
2,3-naphthalenediol |
sodium azide |
dithiothreitol |
glutathione |
borate |
|
tropolone |
methimazole |
glutathione |
natural peptides |
|
|
mimosine |
tropolone |
cysteine |
4-hexylresorcinol |
|
|
benzoic acid |
hydroxamic acid |
thioglycollate |
|
|
|
quercetin |
kojic acid |
|
|
|
|
salicylhydroxamic acid |
maltol |
|
|
|
|
phenylhydrazine |
cyanide |
|
|
|
|
anisaldehyde |
|
|
||
|
(2E)-alkenals |
|
|
|
|