Background Part 1
An Introduction to Proteins
What makes up a cell? If your answer is "mostly water", you're correct! Water makes up roughly 70% of our cells. But what about the other 30%? This section can be divided into small molecules, and macromolecules. These macromolecules, such as nucleic acids, proteins, and polysaccharides, perform many of the reactions necessary for life.
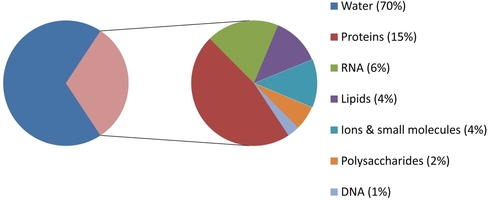
Since biochemistry inherently is the study of biological chemical reactions, these macromolecules are of chief importance to us. We will therefore begin this class with an in-depth look at the largest, most diverse group of macromolecules, proteins.
Unlike DNA, which forms almost exclusively a double stranded, helical conformation, proteins can take on a wide assortment of 3D shapes depending on their amino acid sequence. An example of this variation is shown below, where the structure of the proteins are given in the "cartoon" format. This format simplifies the protein structure to show the two common secondary structures of protein, the alpha helix and beta sheet. These structures play a large role in protein function, allowing proteins to have an incredibly diverse range of functions within the cell.
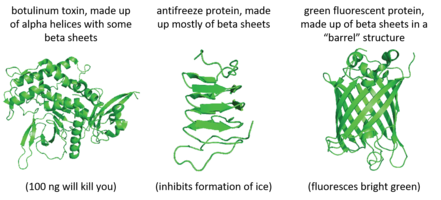
To summarize, the function of a protein is dependent on its 3D structure. The 3D structure is determined by the amino acid composition of the protein.
Enzymes
Recommended reading: Wilson and Walker 23.1 and links on this page.
Lecture Video
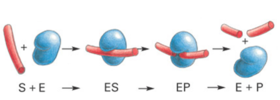
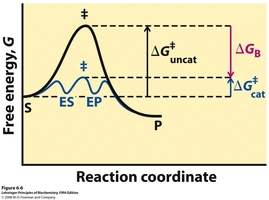 Over the next few weeks we will be working with tyrosinase, a protein enzyme. Enzymes are biological macromolecules that catalyze (i.e., increase the rates of) chemical reactions. Not all enzymes are made or protein (some are made of RNA, see Lehninger ). Recall that proteins are also polymers of amino acids (Lehninger Ch. 3). Proteins have a remarkable variety of properties because they are folded into specific conformations that define a three-dimensional structure (Lehninger Ch. 4, see tyrosinase figure in section below). Catalysts make reactions occur faster by selectively binding a substrate (Lehninger Ch. 5, see below figure) and then lowering the activation barrier of a chemical process (Lehninger Ch. 6, see figure to the right). To lower the activation energy for a reaction, the system must acquire an amount of energy equivalent to the amount by which ΔG‡ is lowered. Much of this energy comes from binding energy (ΔGB) contributed by formation of weak noncovalent interactions between substrate and enzyme in the transition state. Keep in mind that catalysts do not alter reaction equilibria (ΔG of the products and substrates).
Over the next few weeks we will be working with tyrosinase, a protein enzyme. Enzymes are biological macromolecules that catalyze (i.e., increase the rates of) chemical reactions. Not all enzymes are made or protein (some are made of RNA, see Lehninger ). Recall that proteins are also polymers of amino acids (Lehninger Ch. 3). Proteins have a remarkable variety of properties because they are folded into specific conformations that define a three-dimensional structure (Lehninger Ch. 4, see tyrosinase figure in section below). Catalysts make reactions occur faster by selectively binding a substrate (Lehninger Ch. 5, see below figure) and then lowering the activation barrier of a chemical process (Lehninger Ch. 6, see figure to the right). To lower the activation energy for a reaction, the system must acquire an amount of energy equivalent to the amount by which ΔG‡ is lowered. Much of this energy comes from binding energy (ΔGB) contributed by formation of weak noncovalent interactions between substrate and enzyme in the transition state. Keep in mind that catalysts do not alter reaction equilibria (ΔG of the products and substrates).
Tyrosinase
The common names of enzymes are typically derived from their substrate and/or reaction name followed by the suffix "-ase." Two enzymes isolated from different sources and measured for their catalytic activity with different substrates can turn out to be the same protein. Thus, the enzyme tyrosinase, originally discovered in animals, was named for its action on the amino acid tyrosine. It was noted that tyrosinase specifically forms dopaquinone as a product, which is an intermediate metabolite in the production of melanin. The same enzyme isolated from plant materials had been examined for its ability to oxidize phenols, and thus it has historically been reported with other related names such as phenolase, monophenol oxidase and cresolase (cresols are methylphenols). For our purposes, we will use the common name of tyrosinase. The pop-out slideshow below shows three views of a published tyrosinase structure.1 Note the catalytic copper ions. It is important for a biochemist working with an enzyme to have some familiarity with features the active site such as metal ions, catalytic side chains, and cofactors. You will be expected to comment on the structure of the active site in your report (presence/identity/number of Cu ions; relevant active site residues, etc). The primary biological function of tyrosinase is the production of melanin, the ubiquitous brown-black pigment found in animals (e.g. human skin, hair, and eyes), plants (e.g. the browning of potatoes, apples, and avocados), and fungi (e.g. the brown of mushrooms).
The Reactions of Tyrosinase
Tyrosinase is a member of a family of enzymes called polyphenol oxidases (PPO) that play critical roles in enzymatic browning reactions. PPOs are divided into three categories based on the reactions they catalyze. All enzymatic reactions have an enzyme commission (EC) classification that consists of four numbers. The first number indicates the general class of chemical reaction and the additional are more specific subclasses. For example, all enzymes that catalyze oxidation/reduction reactions have a first digit on 1. The EC classifications for the PPOs are EC 1.14.18.1 for tyrosinase, EC 1.10.3.1 for catechol oxidase, and EC 1.10.3.2 for laccase.2 The activity of tyrosinase can be divided into two steps, cresolase and catecholase activities (Scheme 1).
The first reaction, the cresolase activity, inserts an oxygen atom into a monophenol, hence the alternate name monophenol monooxygenase activity. Typically, the amino acid tyrosine is oxidized to generate L-3,4-dihydroxyphenylalanine (L-DOPA). The second reaction, the catecholase activity, oxidizes an ortho-diphenol (also known as a cetechcol) to a quinone. Catechol oxidase enzymes are functional variants of tyrosinase that are structurally related by evolution but lack cresolase activity. For this reason a separate EC identifier (EC 1.10.3.1) is used to designate enzymes with catecholase activity only. Note that both of these reactions use molecular oxygen as electron acceptors. The first reaction adds an oxygen atom to the substrate, the second does not. Instead, it reduces oxygen to water.
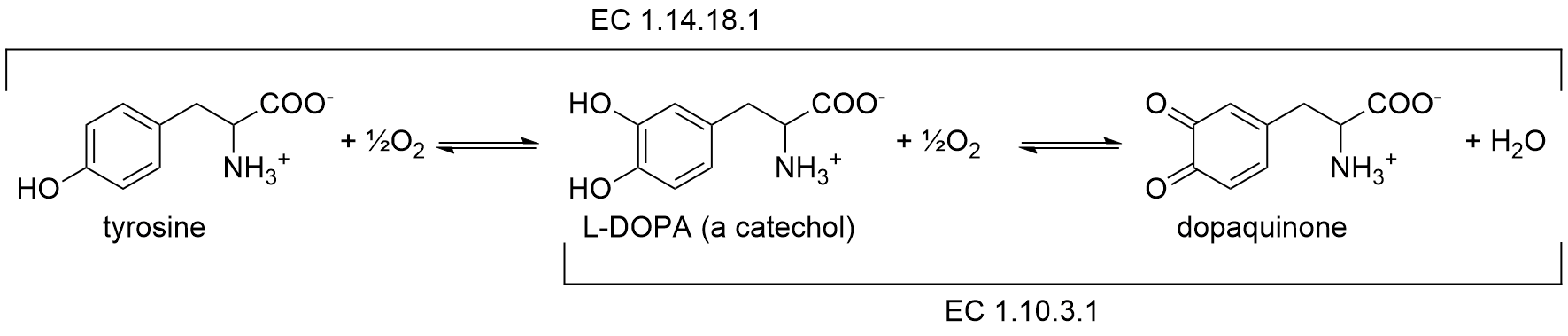
Scheme 1. Tyrosinase catalyzes two oxidations that utilize molecular oxygen as an electron acceptor
Scheme 2 shows a third reaction that generates dopachrome, an unstable precursor compound for the synthesis of melanin, . Most tyrosinases from diverse species can oxidize L-DOPA to yield dopachrome as a colored product, but the extent of dopachrome accumulation depends on the isozyme and assay conditions (pH, presence of reductants/thiols, and competing downstream reactions).6
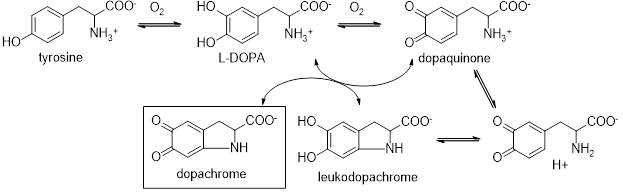
Scheme 2. Steps of tyrosinase catalyzed dopachrome synthesis
The mechanism utilizes substrates in two active sites. In the first site, the enzyme consumes oxygen to react L-Dopa and in the second site, the compound cyclizes and is oxidized by a dopaquinone molecule in the first site as an oxidizing agent to generate leukodopachrome. Thus a second L-Dopa molecule functions as both a substrate and a redox cofactor (much like NADH in other enzymes). You will take advantage of the strong absorbance of dopachrome at 475 nm to both locate tyrosinase in a given tissue and to monitor the kinetics of this enzyme via kinetic assay utilizing L-Dopa as a substrate.
Different Forms of Enzymes
In this lab project, we will characterize the localization of different variants of tyrosinase within the common white mushroom, Agaricus bisporus.
A complication of working with enzymes is that they can exist in different forms. Isozymes or isoenzymes are enzymes with different amino acid sequences (primary structure), but catalyze the same chemical reaction. Isozymes usually display different kinetic parameters (e.g. kcat and different Km values) and/or different regulatory properties. Within a single organism, the varied properties of isoenzymes permit for the fine-tuning of metabolism in order to meet the particular needs of a given tissue or developmental stage (you will learn more about this in CHE 342). In many cases, they are coded for by homologous genes in different loci of the chromosome that have evolutionarily diverged over time and thus have slightly different sequences. It is unknown how many isozymes of tyrosinase exist in the common white button mushroom.
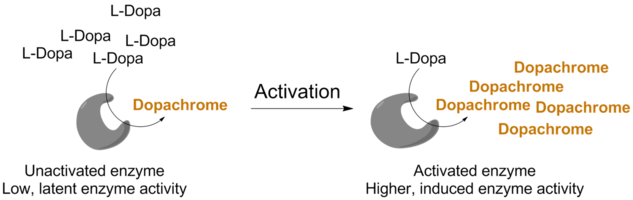
Additionally, enzymes are often expressed in an inactive state that can be "turned-on" by the cell. Tyrosinase from the common white button mushroom exists in different isozyme forms. It is believed to be composed of a single protein subunit (MW 64 kDa) that is hydrolyzed by proteases to generate a smaller, active subunit with a size of ~45 kDa. The 64 kDa enzyme is "latent" and not active. The 45 kDa enzyme is the active form. These 45 kDa subunits associate with a 13 kDa protein whose function is not known. Together these two proteins may associate into dimers (of 116 kDa) and tetramers (of 232 kDa).2,3 In week 3 of this project you will assess the oligomerization state and estimate the molecular weight of tyrosinase isozymes through use of gel electrophoresis.
If this weren't complicated enough, it should be noted that the variants of tyrosinase found in plant, animal and fungal tissue frequently differ with respect to their primary structure (amino acid sequence), size, and activation characteristics.4 However, all tyrosinase isozymes share a common binuclear three copper center within their active site. Here two copper atoms are each coordinated with three histidine residues, see Figure 1.5
Tyrosinase Activation by SDS
In the cell, tyrosinase is activated by hydrolysis catalyzed by an unknown protease.8 Curiously, researchers have reported that very small amounts of the detergent SDS also activates latent tyrosinase. SDS, also known as sodium dodecyl sulfate or sodium lauryl sulfate, is one of the most common synthetic detergent surfactants. It is a primary ingredient in most liquid hand soap, toothpastes, shampoos, and shaving creams. In a biochemistry laboratory, is commonly used in cell lysis to extract DNA and RNA9 and to denature (unfold) proteins in SDS-PAGE experiments used to separate and visualize protein mixtures.10 In the tyrosinase literature, it has been reported to significantly induce the activity of some tyrosinase enzymes at or below the critical micelle concentration of 0.10% (3.5 mM) SDS.8 This was found to decrease sharply at 0.49% (17 mM) SDS.11 In Lehninger Section 4.4, you will learn that large concentrations of SDS cause proteins to denature (unfold) and thus lose all function. It has It is not well understood why small amounts SDS activate tyrosinase, however, you will test to see if the latent enzyme responds differently to SDS activation present in different mushroom tissues.
The Parts of an Agaric Mushroom
Lecture Video
 The common white mushroom, Agaricus bisporus, is a fungus of the order Agaricales. This order contains over 13,000 fungi all of which are readily recognizable as gilled mushrooms.
The common white mushroom, Agaricus bisporus, is a fungus of the order Agaricales. This order contains over 13,000 fungi all of which are readily recognizable as gilled mushrooms.
The above-ground spore-body of agaric fungi all share common features: they are fleshy, with a stipe (stem), a pileus (cap), and lamellae (gills). They often have an annulus (ring) which is a remnant of the veil that protects the gills during maturation.12 The diagram in Figure 3 shows these features in the context of Agaricus bisporus. Based on preliminary histological staining to reveal regions of tyrosinase activity, I have divided the stem into three regions (S1, S2, and S3), the cap into two regions (C1 and C2), the gills are one region (G), and the skin is three regions (K1, K2, and R). These are my divisions. You may decide to group the parts differently for analysis. For example, if your class is small, you may include the ring with the stem or the gills. Each pair of students will analyze an anatomical section. Ideally, the entire mushroom will be analyzed by a single class.